The overall shape of an organic molecule is fixed by the shape of the central carbon atoms, which compose the backbone of the molecule. The shape of this backbone is determined by the types of hybrid orbitals making up the bonds between the central carbon atoms. If the central carbon atoms are sp3 hybridized, the molecule will possess a tetrahedral shape. Central carbon atoms that are sp2 hybridized lead to trigonal-planar shapes, while sp hybridization produces linear molecules. Three-dimensional representations of methane ( sp3 hybridization), ethene ( sp1 hybridization), and ethyne ( sp hybridization) molecules are shown in Figure 1 .

These are examples of structural isomers, or constitutional isomers. Structural isomers have the same molecular formula but a different bonding arrangement among the atoms.
Stereoisomers have identical molecular formulas and arrangements of atoms. They differ from each other only in the spatial orientation of groups in the molecule. The simplest forms of stereoisomers are cis and trans isomers, both of which are created by the restricted rotation about a double bond or ring system. Butene, C4H8, exists in both cis and trans forms
.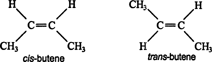

These are examples of structural isomers, or constitutional isomers. Structural isomers have the same molecular formula but a different bonding arrangement among the atoms.
Stereoisomers have identical molecular formulas and arrangements of atoms. They differ from each other only in the spatial orientation of groups in the molecule. The simplest forms of stereoisomers are cis and trans isomers, both of which are created by the restricted rotation about a double bond or ring system. Butene, C4H8, exists in both cis and trans forms
.
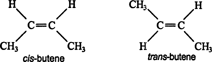
ليست هناك تعليقات:
إرسال تعليق